The world’s first personalized mRNA cancer jab for melanoma – which also has the potential to stop lung, bladder and kidney cancer – is being tested in British patients.
The “gamechanger” shot, which offers hope of a cure, is tailor-made for each person in just a few weeks.
It works by telling the body to detect cancer cells and prevent the deadly disease from returning.
A phase 2 trial of the jab, involving pharmaceutical companies Moderna and MSD, found it dramatically reduced the risk of the cancer returning in melanoma patients.
Now a final phase 3 trial has been launched, led by University College London Hospitals NHS Foundation Trust (UCLH).
Dr. Heather Shaw, national coordinating investigator for the trial, said the jab had the potential to cure people with melanoma and was being tested in other cancers.
Sharing details of the trial exclusively with the PA news agency, she said: “This is one of the most exciting things we have seen in a very long time.
“This is a very finely honed instrument. To be able to sit there and say to your patients that you’re offering them something that’s basically like the Fat Duck at Bray versus McDonald’s – it’s that level of cordon bleu that comes to them.
“These things are extremely technical and sophisticated for the patient. The patients are very enthusiastic about it.”
The new shot is an individualized neoantigen therapy (INT) and is sometimes called a cancer vaccine.
It is designed to activate the immune system so it can fight back against the patient’s specific type of cancer and tumor.

Known as mRNA-4157 (V940), the jab is designed to target tumor neoantigens, which are expressed by tumors in a particular patient.
These are markers on the tumor that may be recognized by the immune system.
The shot encodes up to 34 neoantigens and activates an anti-tumor immune response based on the unique mutations in a patient’s cancer.
To make the shot, a tumor sample is removed from the patient during surgery, followed by DNA sequencing and the use of artificial intelligence.
The result is a personalized anti-cancer injection, specific to the patient’s tumor.
Dr. Shaw said: “This is very much an individualized therapy and in some ways is much smarter than a vaccine.
“It is absolutely tailor-made for the patient. You couldn’t give this to the next patient in line because you wouldn’t expect it to work.
“They may have some shared novel antigens, but they probably have their own, very individual novel antigens that are important to their tumor and that’s why it’s really personalized.”

The ultimate goal is to cure patients of their cancer, Dr. Shaw said.
“Absolutely, that’s the drive. With (this) therapy you are dealing with the theoretical risk that the cancer will come back.
“So nothing shows up on scans, but if there are cells that have escaped that can’t be detected by imaging… what we try to do is, on a patient-by-patient basis, give treatment to eradicate them. those rogue cells that might be hanging out there.
“What we’re trying to do is push more patients into that recurrence-free survival basket, which should translate into an overall survival benefit and non-relapse of those patients over time, which equals a cure.”
Phase 2 data, published in December, showed that people with high-risk severe melanoma who received the shot alongside MSD’s immunotherapy Keytruda were almost half (49%) more likely to die or have their cancer come back after three years than those who received the injection. only Keytruda.
Patients received one milligram of the mRNA vaccine every three weeks for up to nine doses, and 200 milligrams of Keytruda every three weeks for about a year (up to 18 doses).
The global Phase 3 trial will now include a wider range of patients and hopes to recruit around 1,100 people.
The UK arm aims to recruit at least 60 to 70 patients across eight centres, including London, Manchester, Edinburgh and Leeds.
The therapy combination is also being tested in lung, bladder and kidney cancer.
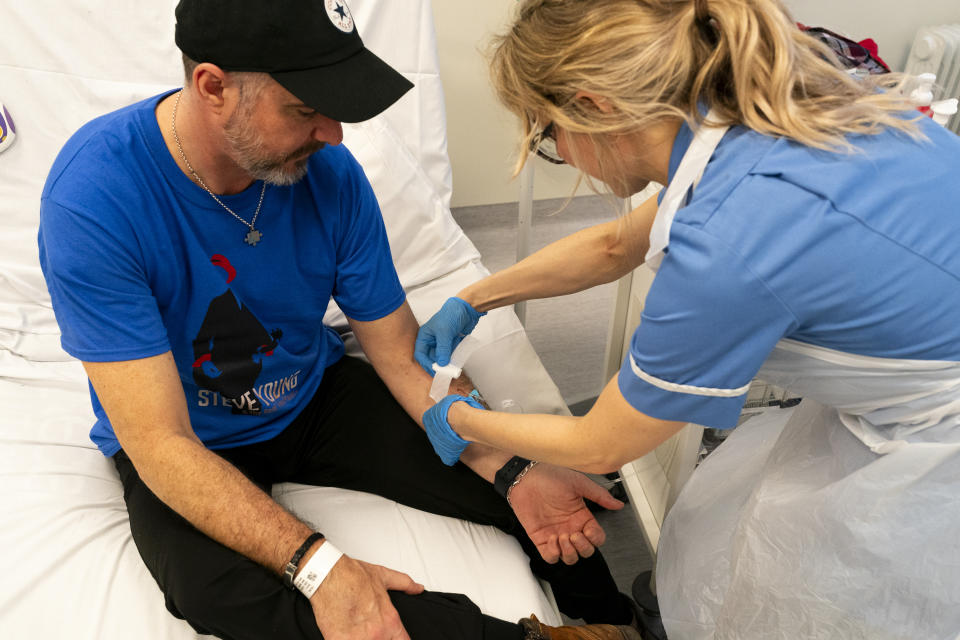
One of the first patients to take part in the trial at UCLH is Steve Young, 52, from Stevenage.
His ‘bump on his head’ – which he thinks he has had for ten years – turned out to be a melanoma.
He told PA it was a “huge shock” to receive the diagnosis.
“I literally spent two weeks thinking, this is it,” he said.
“My father died of emphysema when he was 57 and I actually thought ‘I’m going to die younger than my father’.”
Mr Young said that when he was told about the trial at UCLH it “really triggered my nerd radar”.
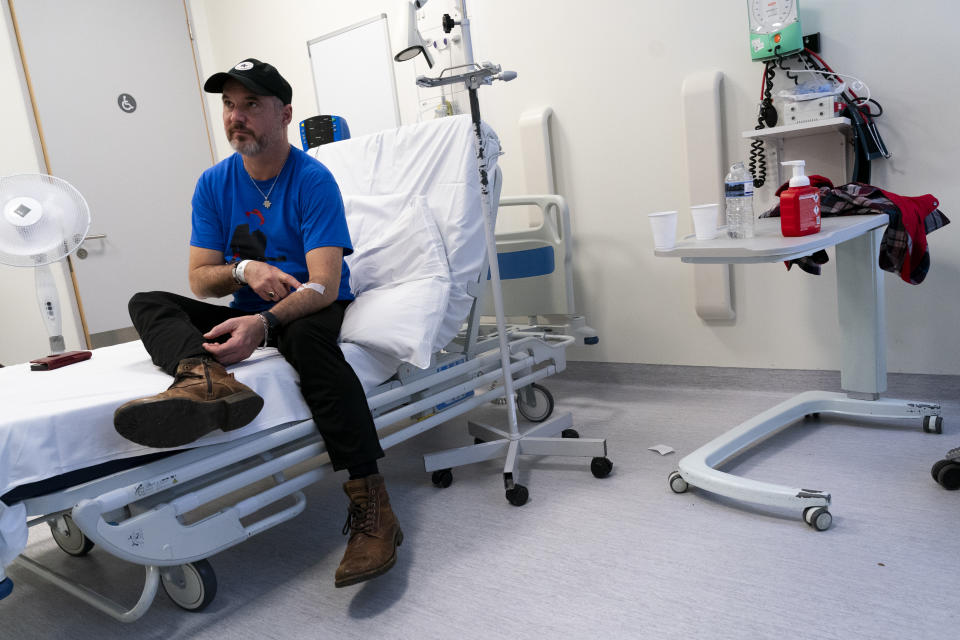

He added: “It really piqued my interest. As soon as they mentioned this mRNA technology potentially being used to fight cancer, I thought, ‘it sounds fascinating’ and I still feel the same way. I’m really excited.
“This is my best chance to stop the cancer.”
Dr. Shaw said: “I think there is real hope that these will be game changers in immunotherapy.
“We have been looking for a long time for something that would complement the immunotherapies we already have – which we know can be life-changing for patients – but with something that has a very acceptable side effect profile.
“And these therapies look like they can offer that promise.”
Dr. Shaw said side effects include fatigue and a sore arm at the site of the shot.
“So it seems relatively tolerable and actually no worse than a flu vaccine or a Covid shot for the majority of patients,” she said.
Professor Lawrence Young from the University of Warwick said: “This is one of the most exciting developments in modern cancer therapy.
“Combining a personalized cancer vaccine to boost a specific immune response to the patient’s tumor, along with using an antibody to release the brakes on the body’s immune response, has already shown great promise in patients at who has had the original skin cancer (melanoma) removed.
“Interest in cancer vaccines has been reignited in recent years by a deeper understanding of how the body controls immune responses and by the advent of mRNA vaccines, which allow developing a vaccine based on the immune profile of the patient’s own tumor patient becomes much easier.
“The hope is that this approach can be expanded to other cancers, such as those of the lung and colon.”
Vassiliki Karantza, associate vice president of MSD Research Laboratories, said: “At MSD, we strive to advance research into innovative modalities at earlier stages of cancer, where we can have the most meaningful impact on patients.
“This study demonstrates our ongoing efforts to advance new treatment options for patients with melanoma and we look forward to expanding our extensive clinical development program to other tumor types.”