The secrets of supernova star explosions could be hidden in dust scattered across the moon – and a team of scientists from the China Institute of Atomic Energy (CIAE) has come up with a new way to unlock those clues to star death.
The research could help scientists get a clearer picture of how stars die and provide material for the next generation of stars, planets, moons and sometimes even life – at least when it comes to Earth.
The technique depends on the improved detection of a rare iron isotope found in infinitesimal amounts in lunar dust. This form of iron was forged millions of years ago in the hearts of previous generations of massive stars. When these stars lost their “tug-of-war” battles with gravity, which lasted millions (or billions) of years, and thus ended their lives in supernova explosions, the isotopes would have been released and spread throughout the cosmos — including, scientists believe, on the moon.
Related: Peer into the remains of an 800-year-old supernova and see a ‘zombie star’
“Our team agreed that the only way to accurately track historical supernova events was to push the limits of what our equipment could do,” team leader and CIAE researcher Bing Guo said in a statement.
How supernovae contribute to cosmic recycling
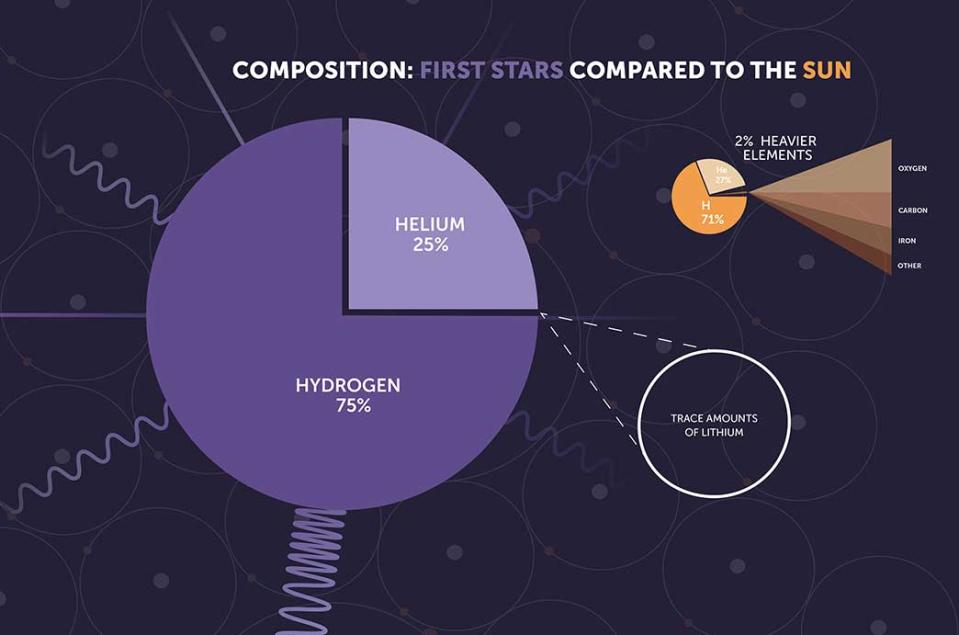
When the first generation of stars was born between 200 million and 400 million years after the Big Bang, the universe was populated largely by hydrogen, with a dash of helium. At that time, there were very few atoms of elements heavier than these, which astronomers (somewhat confusingly) call “metals.”
This means that the first stars, paradoxically called Population III stars, were composed of hydrogen, some helium, and hardly any metals. While these stars lived, the nuclear fusion processes in their cores, which turned hydrogen into helium, allowed them to shine brightly in the cosmos. This fusion process also created the outward radiation pressure that prevented the inward force of their own gravity from causing them to collapse.
However, that meant that when the hydrogen in the cores of these stars ran out, the balancing act between radiation pressure and gravity ended, with the latter being the clear winner. Thus, the cores of these stars collapsed while their outer layers, where nuclear fusion was still taking place, were blown away.
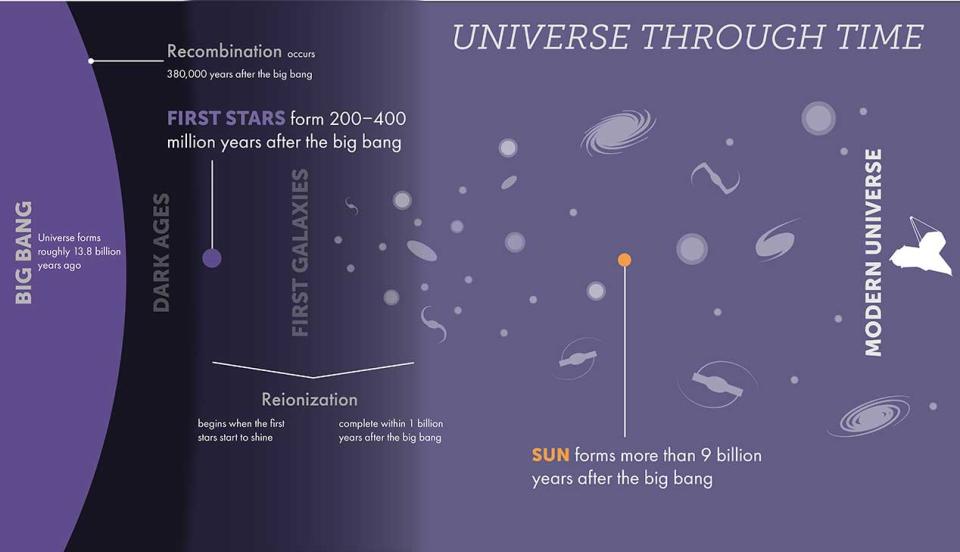
For stars with masses around that of the Sun, this results in their cores becoming white dwarf stars, surrounded by a gradually spreading and cooling cloud of once star material. However, that is not the fate of stars with a mass of at least eight times the Sun.
When these massive stars collapse, the pressure generated in their cores causes the nuclear fusion of helium with other heavier elements. In the most massive stars, this process repeats until the core is filled with iron, the heaviest element a star can ever forge.
After this, the core of a massive star will collapse again and a supernova explosion will occur. That explosion, in turn, releases all the elements the star has generated throughout its life and spreads them throughout the surrounding galaxy. The star then becomes a dense stellar remnant: a neutron star or, in the event of complete gravitational collapse, a black hole.

However, even that isn’t the end for the elements the star forged during his lifetime. These materials find their way into interstellar clouds of gas and dust, which can eventually collapse into birth stars and planets.
That’s how subsequent stellar generations become more and more ‘metal-rich’ as time goes on. All the scattered material is also integrated into nascent planets orbiting these stars and any life forms that might exist on those worlds. So when scientists say, “you’re star people,” it’s more than just lip service; It is a fact.
The moon dust tracking team is interested in a tracer of this cosmic recycling process, which is not an element created during the star’s lifetime, but rather a rare isotope created during a supernova.
Iron-60: from supernovae to the moon
Atoms are composed of three particles: within the atomic nucleus are positively charged protons and neutral neutrons, and in orbit around this nucleus are negatively charged electrons.
Elements are defined by the number of protons in their atomic nucleus. An atom with six protons in the nucleus is therefore always carbon. Add another proton and it becomes a nitrogen atom. However, elements have more flexibility when it comes to the number of neutrons in their nucleus.
A carbon atom can have six protons and six neutrons, or it can have six protons and seven neutrons, or six protons and eight neutrons. These different variations of atoms of the same element are called “isotopes” of that element. A carbon atom with six protons and six neutrons is called “carbon-12”, while carbon atoms with six protons and seven neutrons are the carbon isotope “carbon-14”.
Some of these isotopes, especially the heavier ones, are unstable and undergo a process called radioactive decay. The period over which half a certain amount of a radioactive isotope decays is called a ‘half-life’.
When supernovae erupt within seconds, they release as much energy as it would take for the Sun to radiate in billions of years. This creates the conditions necessary to forge heavy radioactive isotopes. The team is trying to improve ways to hunt for a radioactive isotope of iron called “iron-60” in lunar dust.


Iron-60 has an atomic nucleus containing 26 protons and 34 neutrons, and its half-life is approximately 2.3 million years. However, although a supernova can create iron-60 in amounts equal to about ten times the mass of Earth, the production of this isotope in the solar system is negligible. Scientists predict that supernovae throughout the Milky Way will occur about three times every hundred years, with “nearby” star explosions occurring even less frequently: once every 100 years. million years.
Finding iron-60 on Earth or the moon is a good indicator of a supernova erupting relatively close to the solar system – within about 100 light-years, for example – in the recent history of our 4.6 billion-year-old planet. says the research team.
However, the scarcity of iron-60 and the effect of other more common interfering elements have made detecting its low abundance extremely challenging for low-sensitivity spectrometers. To combat this, Guo and his colleagues made modifications to the CIAE’s HI-13 tandem accelerator facility. This involved adding a ‘Wein filter’, a device that can be used to select charged particles traveling at specific speeds, to perform ‘accelerator mass spectrometry’ (AMS).
The team found that AMS is able to detect iron-60 in simulated samples with sensitivity far beyond what can be achieved with the technology typically used for these studies.
Related stories:
— Aftermath of explosions from two stars captured in a breathtaking new NASA image
— Astronomers catch a rare glimpse of the oldest known supernova, dating back to the year 185
— These supernovae create a storm and contribute to cosmic life and death
The CIAE team believes it is now possible to increase the detection sensitivity of their AMS system even further, a development that could significantly improve our understanding of stars that died in supernova explosions, allowing us to live.
“The installation of the Wien filter could be a game changer for us,” Guo said. “Our next goal is to optimize our entire AMS system to achieve even lower detection limits. Every bit of increased sensitivity opens up a universe of possibilities.”
The team’s research was published May 24 in the journal Nuclear Science and Techniques.