For millions of people, smartwatches are not just a piece of technology. They can use them to take control of their health in ways never thought possible.
As you go for a morning run, a smartwatch can track the rhythmic thumping of your feet and the steady beat of your heart. The watch can record the distance traveled and the intensity of your workout, helping you achieve your fitness goals.
At lunch, you can use it to track calories for a BLT sandwich. As deadlines approach, they can provide gentle reminders to take a moment for yourself. And as you fall asleep, they may notice cases of apnea or other sleep disorders.
But some users may also combine health tips with medical advice. Device and app developers have consistently made it clear that their products cannot replace the advice or treatment of a professional physician.
A smartwatch is not a medical device as defined by law. In Britain, medical devices are strictly regulated in a way that other devices, such as smartwatches, are not. These regulations provide users with better legal protection and clarity and provide solutions in the event of an accident.
What qualifies
The main legal framework in Great Britain is the Medical Devices Regulations 2002 (UK MDR). Once a product is identified as a medical device under the UK MDR, it is further classified, ranging from low risk (stethoscopes and wheelchairs) to high risk (pacemakers, heart valves, implanted brain simulators).
If a device is designed to enter the body, or if it contains medicinal substances, it is more likely to be considered high risk. Depending on the risk classification, the law then imposes strict standards to protect users from harm. These include obligations for manufacturers and developers to ensure that their devices are safe, by conducting risk impact assessments, periodic audits and other actions.
All matters relating to medical devices in Great Britain are the responsibility of the Medicines and Healthcare Products Regulatory Agency (MHRA). The MHRA oversees medical devices available in Britain and has the power to make decisions about their marketing and distribution. It is also the MHRA’s duty to ensure that manufacturers and developers comply with the regulations.
Looking for well-being?
A key question is how to distinguish a device, digital tool or app as one used for medical purposes – as the UK MDR defines a medical device – versus one used for general health and wellbeing. For the latter, you can think of meditation apps or pedometers, for example.
Traditionally, smart watches have been treated as smart, wearable technology. On the surface, they offer users insight into their overall health and wellness, allowing them to make the necessary lifestyle adjustments to improve their health or fitness goals.
However, in recent years, such technologies have become increasingly sophisticated. Tens of thousands of digital tools and applications have flooded app stores. These include mental health monitoring apps, symptom checkers based on information entered by patient users, or medical calculators for drug dosage.
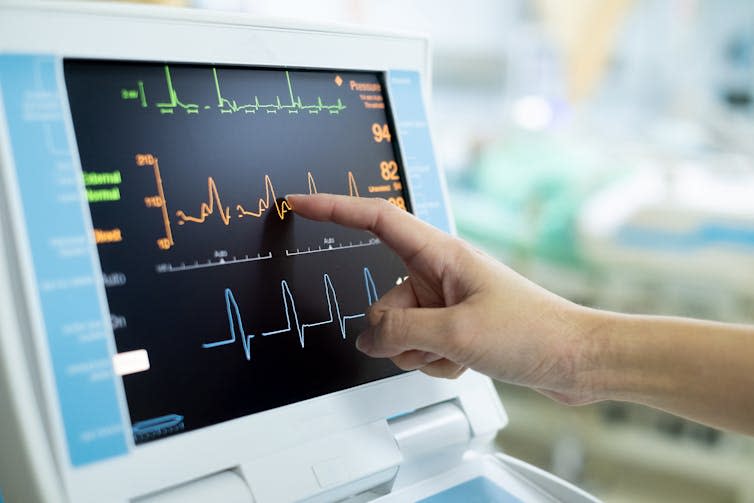
Smartwatches may have electrocardiogram (ECG) functions. An ECG is a test used to check a person’s heart rhythm and electrical activity. Medical professionals traditionally use ECGs to look for signs of coronary artery disease or other cardiovascular conditions. The same features on a watch may not have the right sensitivity to detect medical conditions.
The latest version of the Apple Watch has built-in sensors that may be able to detect atrial fibrillation, a form of irregular heart rhythm. In the US, Apple has received approval from the Food and Drug Administration (FDA) allowing it to be used for this purpose, marking a bold step in regulated medicine and healthcare.
Biosensors, previously thought of as devices administered only in clinical settings, have now evolved by design into thin patches for consumer use. Take the Nix Biosensor device. When paired with Apple Watches, it is designed to measure a user’s optimal hydration level in real time by identifying molecular markers in sweat and determining the loss of fluid and electrolytes (substances that maintain fluid balance inside and outside cells) .
Finally, emerging trends also indicate that more and more women are relying on fertility and cycle trackers in smartwatches and advanced apps. However, there are concerns that users could use the information in place of actual contraception.
So as smartwatches and trackers evolve, it’s possible that they’re approaching the threshold for what authorities might consider a medical device.
Privacy protection
There is also something else to think about. Users of devices and digital tools regularly provide their personal data. Businesses must ensure compliance with the UK General Data Protection Regulation (UK GDPR) and the Data Protection Act 2018 (DPA).
Personal health data constitutes a “special category of data”. This would fall within the scope of Articles 6 and 9 of the UK GDPR and Schedule 1 of the DPA. This means that stricter standards will be imposed on the collection and use of such data (in its processing), which may include an obligation to carry out a comprehensive data impact assessment.
The UK’s privacy watchdog, the Information Commissioner’s Office (ICO), issued a statement on February 8, 2024 reminding all app developers to ensure they protect user privacy following the regulator’s review of menstruation and fertility apps.
Other potential safeguards for user privacy could come from the Medicines and Medical Devices Act 2021 (MMDA), from the appointment of the Patient Safety Commissioner and from the National Health Service (NHS), which can now evaluate digital tools using the digital technology assessment criteria (DTAC).
Clear guidelines in this area are not only necessary, they are absolutely necessary. Without them, we potentially risk both stifling innovation and compromising user care.
This article is republished from The Conversation under a Creative Commons license. Read the original article.


Pin Lean Lau does not work for, consult with, own shares in, or receive funding from any company or organization that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.